Chemistry
States Of Matter
Natural science is one of the branches of science. Chemistry is one of the branches of natural science that discusses structure of matter, their nature and changes. For example. Coal is a matter that contains carbon.
In the mediaeval age, some muslim philosophers of Arab tried to make gold out of cheaper matters like copper, tin and lead. They also tried to get a great mana which would lengthen the life of humans. Though they failed in their original attempts yet they wrote down their experiments. Basically, these were the earliest attempts of systematic study of chemistry or experimentation. These mediaeval Arab experimentation with chemistry was called Alchemy and the philosophers were known as Alchemists.
States of Matter
Q.1. What is matter?
Ans: Anything that has a certain mass and occupies space is matter.
A solid matter has a specific mass, volume and shape. Molecules of all matters have a force of attraction. It is known as inter molecular attraction.
Kinetic theory of Particles:
Every substance is made of small particles which remain together by means of inter molecular force of attraction. These molecules also have kinetic force in them. The attempt to prove the states of substances using inter molecular force of attraction and the kinetic force of the particles is known as the kinetic theory of particles. When the particles inside a substance remain in very high force of attraction, they stay together and cannot move that much. This is the solid state. When heat is applied on such solid substance, the particles start to vibrate. When more heat is applied, the particles lose some of their force of attraction and they start some movement. This state of matter is called liquid. Liquid takes the shape of the container without changing their volume.
When more heat is applied on this liquid state of the substance, the particles receive the heat and the kinetic force in them is increased. This kinetic force is increased to a level where they lose inter molecular force of attraction almost completely and start to more freely. This is the gaseous state. When more heat is applied at this state of the substance, the particles will only increase their speed of movement.
Experiment-1:
In the room temperature, take some pure water in a glass jar. Add a small quantity of solid pink Pottassium permanganate (KMnO4) into it.
We will see after sometime, the KMnO4 grains are dissolving into a pink solution. Indeed, the KMnO4 particles are getting motion and scattering freely in the water. In this case, some solid matter is diffused in water or liquid. The rate of diffusion of solid matters in water is slow, however, if heat is applied, the rate will increase.
Similarly, if we do the experiment of diffusion of Potassium permanganate in hot water, the water will turn into pink solution faster. In this case, the KMnO4 particles will get added motion due to heat and scatter faster. This shows that the presence of heat increases the rate of diffusion of solid. The reason behind it is difference in their atomic mass. A gas with lesser atomic mass will have better diffusion rate. Here ammonia gas (atomic mass 17) has spread faster than hydrogen chloride gas. (atomic mass 36.5).
Atomic mass of H2, He, N2, O2 and CO2 gases are 2, 4, 28, 32 and 44 respectively, since hydrogen is the gas with the least atomic mass, its rate of diffusion is faster than others while CO2's being the most, is the slowest.
Q.2. What is diffusion?
Ans: The tendency of solid, liquid or gas to spread spontaneously and uniformly in any medium is called diffusion.
Experiment-3:
Take a glass tube with both faces open, two cottons. Soak a piece of cotton in heavy hydrochloric acid (HCl), solution and soak the other in ammonium hydroxide (NH4OH) solution. Now close the glass tube fixing each of the cottons to a side of it. Here hydrogen chloride gas from HCl solution and ammonia gas from NH4OH solution will get diffused. Within a few moments, you will see a white smoke filling up the tube. It is ammonium chloride (NH4Cl), produced in the reaction between hydrogen chloride gas and ammonia gas. the white smoke will not be positioned in the centre of hte tube, it will be nearer to the hydrochloric acid solution. That means at a same given time, hydrogen chloride gas gone lesser distance than ammonia. That also proves that ammonia gas spreads faster than hydrogen chloride gas because of its faster rate of diffusion. The reason behind it is difference in their atomic mass. A gas with lesser atomic mass will have better diffusion rate. Here ammonia gas (atomic mass 17) has spread faster than hydrogen chloride gas. (atomic mass 36.5).
Atomic mass of H2, He, N2, O2 and CO2 gases are 2, 4, 28, 32 and 44 respectively, since hydrogen is the gas with the least atomic mass, its rate of diffusion is faster than others while CO2 's being the most, is the slowest.
Burning of a Candle and the three States of Wax:
Wax is a mixture of various hydrocarbons organic compounds made of hydrogen and carbon are known as hydrocarbons. In the burning of a wax, three states of matter can be observed simultaneously. There is a thin liner inside the wax. When we add fire to it, the hydrocarbon particles around the liner melt into liquid. The liquid wax absorbs heat and vaporizes first. Then the vaporized wax starts reaction with oxygen of air and produces CO2, water vapour, light and heat. A portion of the liquid wax remains and turns solid again. Thus, in the presence of heat we see three states of wax.
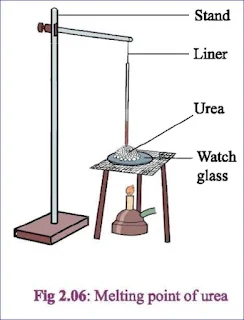
Melting:The process of transforming a solid matter into liquid by means of applying heat is called melting.Melting point:
At normal pressure ( 1 atmospheric pressure), the temperature at which a solid matter turns into liquid state is called the melting point of that solid. Ex. Melting point of ice is 0०C.
Boiling:
The process of transforming a liquid matter into gas by normal pressure (1 atmospheric pressure), the temperature at which a liquid matter turns into gaseous state is called the boiling point of that liquid. Boiling point of water is 100०C.
Boiling point:
At normal pressure (1 atmospheric pressure), the temperature at which a liquid matter turns into gaseous state is called the boiling point of that liquid. Boiling point of water is 100०C.
The process of heating a liquid and turning it into gas is called evaporation or vaporization.
Distillation = vaporization + condensation
The process in which heating a solid directly turns that solid into gaseous substance instead of liquid is called sublimation.Sal ammoniae (NH4Cl)camphor (C10H16O)Naphthalene (C10H8)Solid CO2Iodine (I2)Alumnum Chloride (AlCl3) etc. are matters that do not turn liquid if heat is applied. Instead, they vaporize. These sunstances are called sublimated substances.
Q.1. What kind of action takes place when hot tea is kept in a tea cup?a. vaporization✔b. sublimationc. diffusiond. effusionQ.2. What will happen to the particles when water vapour is condensed?a. size will reduceb. will remain in movementc. will vibrate at the same positiond. energy will evolve✔Q.3. Which of the following pictures is applicable for sublimation?
5. Which of the following has the highest rate of diffusion?a. CO2 b. NH3 c. HCl d. H2SO4
6. Which of the followings can be sublimated?a. Iodine b. edible salt c. Copper sulfate d. Soda ash
Q.1. What is diffusion?
Ans: The tendency of solid, liquid or gas to spread spontaneously and uniformly in any medium is called diffusion.
2.a. Effusion:
The passage of gasses from a high pressure zone to a low pressure zone through a fine pore is called effusion.
3. A beaker containing some ice cubes is slowly heated. With the passage of time, the changes in the state of ice are observed.
a. What is distillation?
Ans: The process of heating a liquid into vapour and then retrieving the liquid from the vapour by cooling it is called distillation.
b. What do you understand by diffusion and effusion?
The tendency of solid, liquid or gas to spread spontaneously and uniformly in any medium is called diffusion.
Effusion:
The passage of gasses from a high pressure zone to a low pressure zone through a fine pore is called effusion.
c. Present the action of the stem on a graph sheet.
d. What will happen if you replace ice of the stem with naphthalene? Analyze.