Chemical Reaction
A chemical reaction is a process in which substances, called reactants, undergo a transformation to form new substances, called products. This change involves the breaking and forming of chemical bonds, often accompanied by energy changes in the form of heat, light, or sound.
Q. What happens in a chemical reaction?
Chemicals react to change
The "chemicals" in a chemical reaction are elements or compounds.
They "react" when combined or broken down.
Q. Do you remember the difference between an element, a compound, and a substance?
- Explain the law of conservation of mass
- Identify parts of a chemical reaction
- Classify chemical reactions according to type
Atoms in a Chemical Reaction:
They rearrange.
Some atomic bonds are broken, and some new ones are made.
They are neither created nor destroyed.
This is the law of conservation of mass. In a chemical reaction, the total mass of the substances before and after the reaction remains the same.
The quantity of elements and their atoms remain the same before and after a chemical reaction.
A chemical equation tells the story of a chemical reaction.
An element is a pure substance and cannot be broken down into a new one.
A compound is a combination of pure substances.
Tell the story of the chemical reaction earlier.
Q. Who are the characters?
Q. How did the "story" begin and end?
Share your version to a seatmate.
Four atoms of hydrogen react with two atoms of oxygen. They are the reactants. They yielded two atoms of water. Water is the product or end result.
A chemical reaction can be a decomposition, synthesis, single-replacement, or double-replacement.
Types of Chemical Reaction:
1. Decomposition: one compound breaks down into two products.
AB -------> A + B
2. Synthesis: two or more substances join and form one single substance.
A + B ------> AB
3. Single-replacement: atoms of an element swap with atoms of a second element in a compound.
AB + C -----> AC + B
4. Double-replacement: positive ions exchange between two compounds.
AB + CD -----> AD + CB
Q. What real-life chemical reactions do you often encounter?
Name a few and share them next time as we learn more about each type
of chemical reaction. See you!
Chemical Symbols and Formulae
Understanding the Language of Chemistry!
What do they mean?
Think of where you might have seen these symbols outside of the classroom.
Today, you will...
Represent elements using chemical symbols.
Understand the basics of IUPAC nomenclature in naming compounds.
Use chemical formulae to represent compounds.
Did You Know?
Swedish scientist Jöns Jakob Berzelius introduced the modern system of chemical symbols.
He represented the elements using one or two letters from their latin names.
Chemical Symbols:
These are shorthand ways to represent elements in chemistry.
Recall that an element cannot be broken down into simpler substances.
Each symbol is made up of one or two letters.
Always capitalise the first letter!
Na
Sodium
Importance of Chemical Symbols:
They provide standardised ways of representing elements, making communicating information about elements and compounds easier.
They offer a concise and simple way to represent elements in chemical equations, eliminating the need to spell each element's name.
Common Chemical Symbols:
H
Hydrogen
- Derived from the Greek ‘hydro’ and ‘genes’, which means water forming
- Most abundant element in the universe
O
Oxygen
- Name comes from the Greek ‘oxy’ and ‘genes’, which means acid forming
- Essential for life
C
Carbon
- Derived from the Latin ‘carbo’, meaning charcoal
- Found in all known life forms
Common Chemical Symbols:
N
Nitrogen
- Comes from the Greek ‘nitron’ and ‘genes’, meaning nitre forming
- Constitutes about 78% of Earth's atmosphere
Na
Sodium
- Derived from the Latin ‘natrium’
- Soft metal that easily tarnishes when exposed to air
Cl
Chlorine
- Name derived from the Greek ‘chloros’, which means greenish yellow
- Widely used in disinfectants and cleaning products
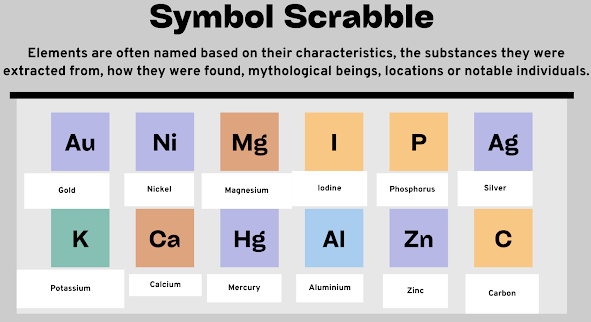
Symbol Scrabble:
How many elements can you recognise from the chemical symbols below?
Name as many as you can with the help of your peer.
Elements are often named based on their characteristics, the substances they were extracted from, how they were found, mythological beings, locations or notable individuals.
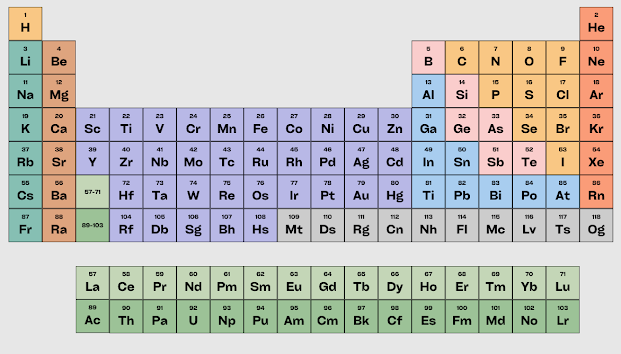
The Periodic Table of Elements:
There are 118 chemical elements listed in the periodic table in a specific order.
Rules on how to name new elements in the periodic table are set up by the International Union of Pure and Applied Chemistry (IUPAC).
The Role of IUPAC:
IUPAC is responsible for establishing unambiguous and consistent naming systems (also known as nomenclature) and terminology.
It has a system for naming chemical compounds that provides a standard language for scientists worldwide.
Naming Compounds:
A compound is a substance containing two or more elements that are chemically bonded.
A chemical formula is an expression showing the elements in a compound and their relative proportions.
It contains a combination of chemical symbols and numbers.
The chemical formula for carbon dioxide is CO₂.
What does the formula CO₂ mean?
Carbon dioxide is a compound of carbon and oxygen.
one carbon atom
two oxygen atoms because of the subscript ‘2’.
Shown below is the molecular model of carbon dioxide.
CO₂
Naming Ionic Compounds:
An ionic compound is composed of a metal and non-metal. Some of the basic rules when naming ionic compounds include:
A metal will always come first in a chemical formula, followed by a non-metal.
Ex: sodium chloride (NaCl)
An ionic compound with two elements will have a name that ends with -ide.
Ex: copper sulfide
(CuS)
A compound with three elements, including oxygen, will have a name that ends with -ate
or -ite.
Ex: sodium sulphate
(NaSO₄)
Naming Covalent Compounds:
A covalent compound has two or more non-metals. Some of the general rules when naming covalent compounds are:
The non-metal furthest to the left on the periodic table comes first.
Ex: carbon dioxide
(CO₂)
A covalent compound with two elements will have a name that ends with -ide.
Ex: sulfur dioxide
(SO₂)
Use the prefixes (mono-, di-, tri-, tetra-) to indicate the number of that element in the molecule.
Ex: carbon tetrachloride
(CCl₄)
Review:
Chemical Symbols: These are shorthand ways to represent elements in chemistry. Each symbol is made up of one or two letters, with the first letter always capitalised.
IUPAC Nomenclature: IUPAC is responsible for setting up a system of naming elements and compounds, providing a standard language for scientists worldwide.
Naming Compounds: Compounds are represented by chemical formulae, which use symbols and subscripts.
IUPAC sets rules for naming compounds correctly.
Chemical Formulae Hunt:
Identify and record the chemical formulae, common names, and corresponding IUPAC names for five (5) common compounds found at home.
Periodic Table
Periodic table is the outstanding collection of chemical concepts gathered over hundreds of years. Various scientists have given their tireless effort after it to get the present state of the table.
Characteristics of the Periodic Table:The periodic table is basically a table of elements. It also features columns and rows. In the periodic table, the rows from left to right are called Periods and vertical columns are called Groups.
The modern periodic table has some prominent characteristics.a) There are seven periods (horizontal rows) and 18 groups (vertical columns) in the periodic table.b) All periods start with group 1 at the extreme left and extend up to group 18 at the extreme right.c) A small table of 2 horizontal rows and 14 columns displays the lanthanide and actinide elements beneath the main periodic table. These are part of period 6 -7 of main periodic table.d) Period 1 contains only two elements. Periods 2 and 3 contain four elements each. Periods 4 and 5 contain 18 elements each. Periods 6 and 7 contain 32 elements each.e) Group 1 contains 7 elements. Group 2 contains 6 elements. Group 3 has 32 elements. Groups 4 to 12 contain 4 elements each. Groups 13 -17 have 6 elements each. Group 18 contains 7 elements.Fifteen elements with atomic numbers 57 to 71 are called Lanthanide elements. Fifteen elements with atomic numbers 89 to 103 are categorized as Actinide elements.Now consider the periodic table in terms of properties of elements.1. Properties of elements change from left to right in the same period.2. The physical and chemical properties of elements of the same group are almost similar.
Determining the Period Number:the number of the outermost main energy level in the electronic configuration of an element is the period number of that element.For example, the electronic configuration of Lithium is Li(3) --> 1s²2s¹. Since the outermost energy level of Lithium is the second one, so the element belongs to the period 2 of the table.
Some Exceptions of the Periodic Table:
a. The Position of Hydrogen:
Hydrogen is a non-metal. However, the periodic table displays hydrogen alongside strong electropositive alkali metals like Na, K, Rb, Cs, Fr etc. in group 1. It is because the outer shell of H contains only 1 electron like the alkali metals. Besides, many properties of hydrogen are similar to those of alkali metals. On the other hand, hydrogen also is able to accept an electron like the halogen elements (F, Cl, Br, I), meaning it has some similarities with Halogen in terms of properties. However, since most of the properties of hydrogen in terms of properties. However, since most of the properties of Hydrogen have similarity with alkali metals, it is placed alongside alkali metals.
b. The Position of Helium
c. The Position of Lanthanide and Actinide Groups
Exploring how elements are arranged throughout the Periodic Table.
- Features of the periodic table
- Electronic structure of an atom
- Properties of metals, non-metals and metalloids
- Periodic trends in reactivity
How are elements arranged in the periodic table?
- Explain the features of the periodic table of elements
- Draw the electronic structure of elements with the help of the periodic table
- Describe the characteristics of metals, non-metals, and metalloids
- Recognise and predict properties based on trends in the periodic table
Periodic Table of Elements:
It contains the atomic mass, atomic number and chemical symbol associated with a known element. As of today, there are 118 elements in the periodic table.
Features of the Periodic Table:
A row is called a period; each element’s atomic number increases as you move along the periods.
Features of the Periodic Table:
A column is called a group; elements in the same group all have the same number of valence electrons in their outer shells.
Now, we’ll study the structure of the atom ‘beyond’ what we can see in the periodic table.
We will look at the element’s electronic structure.
Electronic Structure
Illustrates the electron arrangement of an atom of an element
Electrons occupy energy levels, also known as electron shells.
Electrons can be shown as a cross or a dot.
Electronic Structure:
Follow the rules below when drawing the electronic structure of an element:
- 2 electrons in the first shell
- 8 electrons in the second shell
- 18 electrons in the third shell
- 32 electrons in the fourth shell
- Oxygen
Atomic number = 8
Always start with the first shell and work your way outwards.
Note that only 2 electrons can be on the 1st shell.
Oxygen still has 6 more electrons, we can place that on the 2nd shell since it can have 8 electrons in total.
Electronic Configuration:
Identifies how many electrons are in a shell.
Follow this format in writing electronic configuration:
[1st shell electrons].[2nd shell electrons].[3rd shell electrons] and so on...
Each dot or comma separates one shell from the next.
By following the format, oxygen’s electronic configuration is:
Draw the electronic structure for lithium, sodium and potassium and write the electronic configuration for each element.
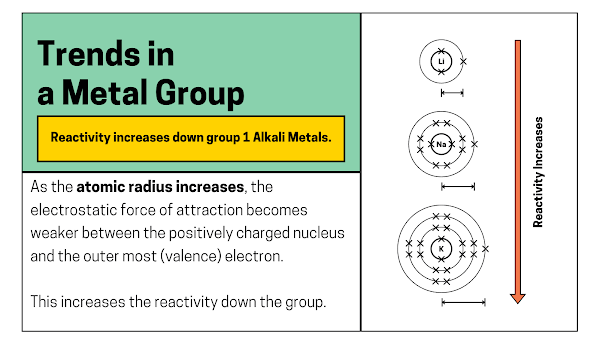
What trend do you notice regarding the atomic number and the electron in the outermost shell?
- The atomic number increases down the group.
- The atomic number tells you how many electrons each element has.
- Each group 1 element structure has 1 electron in its outer shell.
- Elements are grouped together by the number of electrons that they have in their outer shells.
- The modern periodic table organises elements by atomic number, revealing their structural arrangement.
- Elements' positions in the periodic table reflect their atomic structure and outer electron arrangement.
- Similar properties among elements within a group on the periodic table can be explained through identifiable trends.
- Metals and non-metals exhibit characteristic properties that can be identified through their placement on the periodic table.
A covalent bond is formed when two atoms share a pair of electrons. The electrons which contribute towards a covalent bond, are found in the outer shells of the atoms. Usually each atom contributes one electron, but some atoms can react to make multiple covalent bonds.
Water is made of two hydrogen atoms and one oxygen atom. Oxygen has 6 electrons in its outer shell and needs to achieve 8 to make a full outer shell. Each hydrogen has 1 electron and needs to achieve 2 to have a full shell.
Two covalent bonds can be formed to make the simple covalent water molecule.
Fluorine has 7 electrons in its outer shell and needs to achieve 8 to have a full outer shell. Hydrogen has one electron. As this electron is in the first shell, hydrogen needs to achieve 2 electrons to have a full shell. The simple covalent molecule of hydrogen fluoride is made by sharing electrons.
The shells must be filled in order of closest to the nucleus, to furthest from the nucleus. When reacting, the aim is for an atom to achieve a full outer shell. This means the desired electron configuration is the same as a noble gas e.g. like helium and neon shown to the left.
Reactants are found on the left hand side of the equation. Products are on the right hand side of the equation. The reactants and products are separated by an arrow. Scientists write equations as word equations or symbol equations. Symbol equations provide more detail as they show the number of atoms in each reactant or product.
The chemical formula of a compound gives us information about the elements involved and the number of atoms of those elements. The little subscript numbers refer to the element before them. If there is just one atom of an element in the formula, the number 1 is not written.
A state symbol is used in a chemical equation to show if a substance is a solid, a liquid, a gas, or an aqueous solution. For example, there are three gases and one liquid in this reaction:
In chemical reactions, the atoms in the reactants are rearranged to form the products. Atoms can not be lost or destroyed. We balance equations to show this. There should be an equal number of atoms on both sides of the equation. We add ‘big’ numbers at the start of the reactant or product in order to balance the equation.
Ionic compounds can react when they are dissolved in water. Whilst some ions react, ions known as ‘spectator ions’ remain unchanged and are not involved in the reaction. The two types of ionic equation are neutralisation and displacement reactions.
ISOTOPES
ATOMIC STRUCTURE & PERIODIC TABLE
Q.1. Describe what an isotope is.Q.2. Give examples of isotopes.Q.3. Calculate the relative atomic mass of an element.
Q. What are isotopes?
Ans: Isotopes are atoms of the same element with different numbers of neutrons (they have the same number of protons).
Example-1:
There are three isotopes of hydrogen. All hydrogen atoms contain one proton (and one electron), but they can have different numbers of neutrons. Hydrogen-1 is the most commonly found isotope of hydrogen.
Example-2:
There are two isotopes of chlorine. All chlorine atoms contain seventeen protons (and seventeen electrons), but they can have different numbers of neutrons. Chlorine-35 is the most commonly found isotope of chlorine. The other isotope is chlorine-37.
Properties:
Isotopes have the same chemical properties. This is because the number of electrons determines chemical properties, isotopes have the same number of electrons in their atoms.
RELATIVE ATOMIC MASS:
The relative atomic mass of an element in the periodic table, is the weighted average of the masses of the atoms of the isotopes. Relative atomic mass takes into account the abundance of each of the isotopes of the element.
The relative atomic mass of chlorine is 35.45 rather than a whole number. This is due to chlorine having two isotopes (chlorine-35 and chlorine-37).
CALCULATING THE RELATIVE ATOMIC MASS:
Copper has two isotopes:
Copper-63 which accounts for 69% of copper
Copper-65 which accounts for 31% of copper
Calculate the relative atomic mass of copper to one decimal place.
(69 X 63) + (31 X 65) = 6362
6362 / 100 = 63.6
What is relative atomic mass?
Ans:It is the mean relative mass of the atoms of the different isotopes in an element. It is the number of times heavier an atom is, compared to one-twelfth of a carbon-12 atom. It is also equal to the number of protons and neutrons in the atom.
The relative atomic mass of oxygen can be found next to the chemical symbol on the periodic table. It is 16.00.
What is relative atomic mass?
It is the mean relative mass of the atoms of the different isotopes in an element. It is the number of times heavier an atom is, compared to one-twelfth of a carbon-12 atom. It is also equal to the number of protons and neutrons in the atom.
The relative atomic mass of oxygen can be found next to the chemical symbol on the periodic table. It is 16.00.
Q.What is relative formula mass?
The relative formula mass is the sum of the relative atomic masses of the atoms in a chemical formula.
The relative formula mass of hydrogen chloride (hydrochloric acid) is
1.008 + 35.45 = 36.458
The Relative Formula Mass of Water:
There are 2 hydrogen atoms and 1 oxygen atom in a molecule of water.
The calculation:
(2 X 1.008) +(1 X 16.00)
= 18.016
There is one atom of nitrogen and three atoms of hydrogen in a molecule of ammonia.
14.01 + (3 X 1.008) = 17.034
There are 6 atoms of carbon, 12 atoms of hydrogen and 6 atoms of oxygen in a molecule of glucose.
(12.01 X 6) + (1.008 X 12) + (16.00 X 6)
= 72.06 + 12.096 + 96
= 180.156
Electrolysis of molten salts:
# State the products of electrolysis for different electrolytes.
# Describe the movement of ions to the electrodes.
Q.1. What is electrolysis?
Electrolysis is the breakdown (also known as decomposition) of a compound using an electrical current.
An electrolyte is a substance which conducts electricity when molten or in solution. Electrolytes contain free moving ions which are attracted to the oppositely charged electrodes. The electrodes connect to a dc supply.
The cathode is the negatively charged electrode in electrolysis. Positively charged ions move towards the cathode.
The anode is the positively charged electrode in electrolysis. Negatively charged ions move towards the anode.
Positively charged calcium ions (2+) move to the negative electrode, where they gain electrons to form calcium atoms. Negatively charged chloride ions (1-) move to the positive electrode, where they lose electrons, become atoms and pair up to form chlorine molecules.
Molten lead bromide is an electrolyte. During electrolysis, positive (2+) lead ions gain electrons at the cathode and become Pb atoms. Negative (1-) bromide ions lose electrons at the anode and become Br atoms, which pair up to form bromine molecules. Lead forms at the negative electrode and bromine forms at the positive electrode.
You're welcome!
If there's anything else you need help with, feel free to ask.