Enzymes
Cambridge AS level Biology
You should be able to:
1. Explain how enzymes work
2. Describe and explain the factors that affect enzyme activity
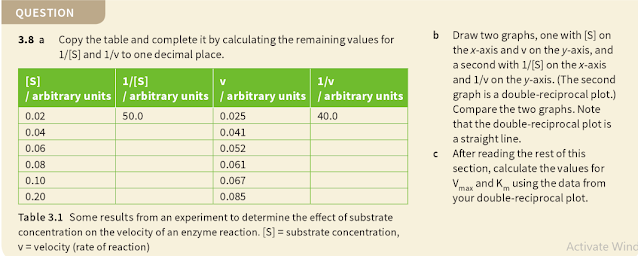
3. Use Vmax and Km to compare the affinity of different enzymes for their substrates
4. Explain how reversible inhibitors affect the rate of enzyme activity
5. Carry out experiments with enzymes under controlled conditions
6. Explain the advantages of using immobilised enzymes
Q/A:
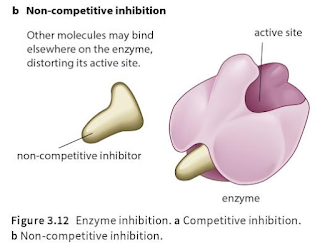
Q.1. The diagram below shows an enzyme and two inhibitors of the enzyme, X and Y. Which of the following describes the two inhibitors?
A. X and Y are competitors inhibitors.B. X and Y are non- competitive inhibitors.C. X is a competitive inhibitor and Y is a non-competitive inhibitor.D. X is a non-competitive inhibitor and Y is a competitive inhibitor.
Q. 2. In a reaction controlled by an enzyme, which of the following graphs shows the effect of substrate concentration on the rate of the reaction?
Ans: D
Q.3. The graph shows the progress of the digestion of starch by the enzyme salivary amylase. Why does the reaction slow down?
A. End-product inhibition by maltose.B. The salivary amylase is becoming denaturedC. The salivary amylase is gradually becoming saturated with starchD. There are fewer and fewer substrate molecules left to bind with the salivary amylase.
Q. 4. If methylene blue dye is added to a suspension of yeast cells, living cells do not take up the stain, and they remain colourless. However, dead cells are strained blue. This fact was used to carry out an investigation into the rate at which yeast cells were killed at two different temperatures (at high temperatures the yeast enzymes will be denatured). The results are shown in the diagram below.
Which of the following is correct?
The higher temperature is The vertical axis (y-axis) should be labelled
A. X % coloured cells
B. Y % coloured cells
C. X % coloured cells
D. Y % coloured cells
Q.5. Copy the graph in question 3 and draw a line from which the initial rate of reaction could be calculated.
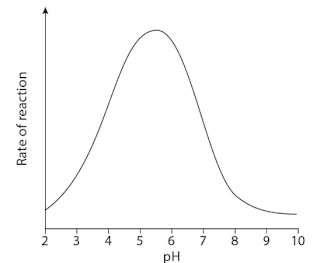
Q. 6. The graph shows the effect of changes in pH on the activity of the enzyme lysozyme.
a. Describe the effect of pH on this enzyme.
Ans: Most enzymes work fastest at a pH of somewhere around 7 - that is, in fairly neutral conditions. Some, however, such as the protease pepsin, which is found in the acidic conditions of the stomach, have a different optimum pH.
b. Explain why pH affects the activity of the enzyme.
Ans: pH is a measure of the concentration of hydrogen ions in a solution. The lower the pH, the higher the hydrogen ion concentration. Hydrogen ions can interact with the R groups of amino acids - for example, by affecting ionisation (negative and positive charges) of the groups. This affects the ionic bonding between the groups which in turn affects the three-dimensional arrangement of the enzyme molecule. The shape of the active site may change and therefore reduce the chances of the substrate molecule fitting into it. A pH which is very different from the optimum pH can cause denaturation of an enzyme.
When investigating pH, you can use buffer solutions. Buffer solutions each have a particular pH and maintain it even if the reaction taking place would otherwise cause pH to charge. You add a measured volume of the buffer to your reacting mixture.
Q.7. The graph below shows the effect of temperature on the rate of reaction of an enzyme.
a. What is indicated by X?
Ans: X indicated energy becoming denatured.
b. What temperature would X be for a mammalian enzyme?
Ans: the temperature at which an enzyme catalyses a reaction at the maximum rate is called the optimum temperature. 40०C temperature would X be for a mammalian enzyme.
c. Explain what is happening in region A.
Ans: At low temperatures, the reaction takes place only very slowly. this is because molecules are moving relatively slowly. As temperature continues to increase, the speed of movement of the substrate and the enzyme molecules also continues to increase.
d. Explain what is happening in region B.
Ans: It would be dangerous to maintain a body temperature of 40० C, as even a slight rise above this would begin to denature enzymes.
e. Enzymes are effective because they lower the activation of the reactions they catalyse.
Ans: The substrate molecule fits less well into the active site of the enzyme, so the rate of the reaction begins to slow down. Eventually, the substrate no longer be held in the correct position for the reaction to occur.
Q. 8. The reaction below occurs during aerobic respiration. The reaction is catalysed by the enzyme succinate dehydrogenase.a. Name the substrate in this reaction.
b. The molecule malonic acid, which is shown here, inhibits this reaction.It does not bind permanently to the enzyme. Describe how malonic acid inhibits the enzyme succinate dehydrogenase.
c. Heavy metals such as lead and mercury bind permanently to - SH groups of amino acids present in enzymes. These -SH groups could be in the active site or elsewhere in the enzyme.
i. Name the amino acid which contains - SH groups.
ii. Explain the function of -SH groups in proteins and why binding of heavy metals to these groups would inhibit the activity of an enzyme.
iii. What type of inhibition would be caused by the heavy metals?
Q. 9. You are provided with three solutions: A, B and C. One solution contains the enzyme amylase, one contains starch and one contains glucose. Starch is the substrate of the enzyme. The product is the sugar maltose. You are provided with only one reagent, Benedict's solution, and the usual laboratory apparatus.
a. Outline the procedure you would follow to identify the three solutions.
b. What type of reaction is catalysed by the enzyme?
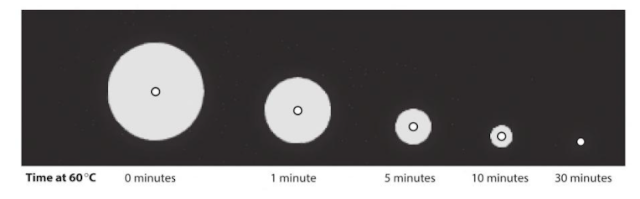
Q.10. The activity of the enzyme amylase can be measured at a particular temperature by placing a sample into a Petri dish containing starch-agar. Starch - agar is a jelly containing starch. One or more 'wells' (small holes) are cut in the agar jelly with a cork borer, and a sample of the enzyme is placed in each well. The enzyme molecules then diffuse through the agar and gradually digest any starch in their path. At the end of the experiment, iodine in potassium iodine solution is poured over the plate. Most of the plate will turn blue-black as iodine reacts with starch, but a clear 'hola' will be seen around the well where starch has been digested. Measuring the size of the halo can give an indication of the activity of the enzyme.
A student decided to investigate the rate at which a mammalian amylase is denatured at 60⁰ C. She heated different samples of the enzyme in a water bath at 60⁰ C for 0, 5, 10, 30 minutes. She then allowed the samples to cool down to room temperature and placed samples of equal volume in the wells of five starch-agar plates, one plate for each heating period. She then incubated the plates in an oven at 40 degree C for 24 hours.
The results of the student's experiment are shown on the next page. A diagram of one dish is shown, and the real size of one halo from each dish is also shown.
a. Why did the student cut four wells in each dish rather than just one?b. One dish contained samples from amylase which was not heated. This is a control dish. Explain the purpose of this control.c. Explain why the starch-agar plates were incubated at 40᠐ C and not room temperature.d. Describe why was happening in the dishes during the 24 hours of incubation.e. Why was it important to add the same volume of amylase solution to each well?f. Measure the diameter in mm of the representative halo from each dish. Record the results in a suitable table.g. Only one halo from each dish is shown in the diagrams. In practice there was some variation in the diameters of the four halos in each dish. How would you allow for this when processing your data?h. Plot a graph to show the effect of length of time at 60० C on the activity of the enzyme.i. Describe and explain your results.
j. Another student that amylases from fungi and bacteria are more resistant to high temperatures than mammalian amylases. Using starch-agar plates as a method for measuring the activity of an amylase at 40⁰ C, outline an experiment that the student could perform to discover which amylase is most resistant to heat. Note that temperatures up to 120⁰ C can be obtained by using an autoclave.k. Enzymes are used in many industrial processes where resistance to high temperatures is an advantage. State three other variables apart from temperature which should be controlled in an industrial process involving enzymes.
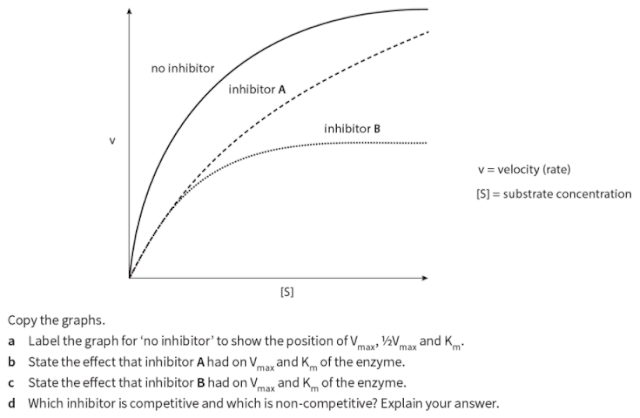
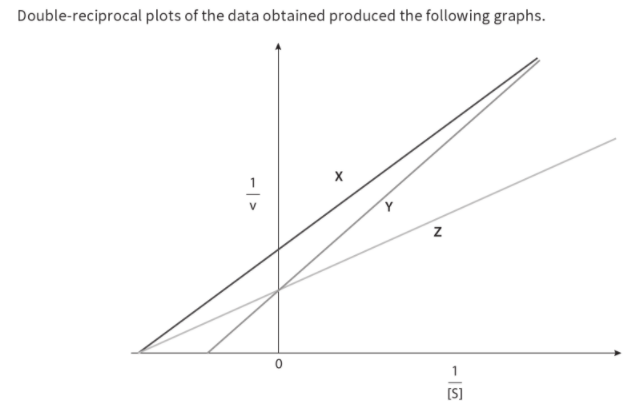
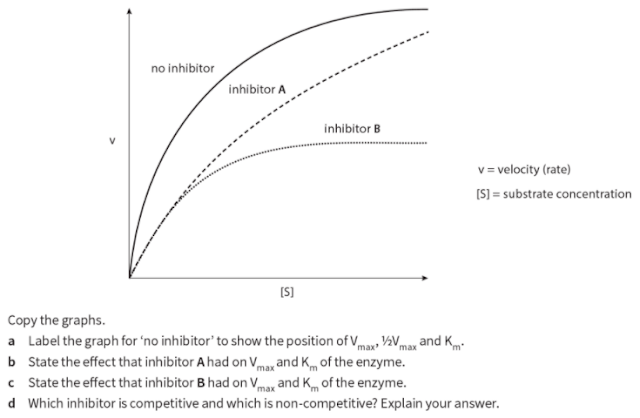
Q. 11. Two inhibitors of the same enzyme, inhibitor A and inhibitor B, were investigated to discover if they were competitive or non-competitive. In order to do this, the rate of reaction of the enzyme was measured at different concentrations of substrate without inhibitor, with inhibitor A and with inhibitor B. Graphs of the data were plotted as shown on the next page. The graphs showed that one inhibitor was competitive and the other non-competitive.
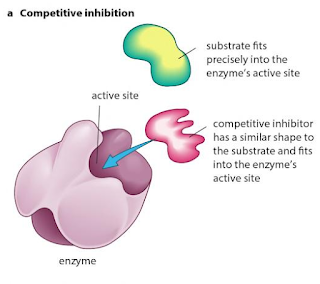
Competitive inhibition:When a substance reduces the rate of activity of an enzyme by competing with the substrate molecules for the enzyme's active site; increasing the concentration of the substrate reduces the degree of inhibition.
Non- competitive inhibition :When a substance reduces the rate of activity of an enzyme, but increasing the concentration of the substrate does not reduce the degree of inhibition; many non-competitive inhibitors bind to areas of the enzyme molecule other than the active site itself.
Competitive, reversible inhibition:If an inhibitor molecule binds only briefly to the site, there is competition between it and the substrate for the site. If there is much more of the substrate present than the inhibitor, substrate molecules can easily bind to the active site in the usual way, and so the enzyme's function is unaffected. However, if the concentration of the inhibitor rises, or that of the substrate falls it becomes less and less likely that of the substrate will collide with an empty site. The enzyme's function is then inhibited. This is therefore known as competitive inhibition. It is said to be reversible because it can be reversed by increasing the concentration of the substrate.
An example of competitive inhibition occurs in the treatment of a person who has drunk ethylene glycol. Ethylene glycol is used as antifreeze, and is sometimes drunk accidentally. Ethylene glycol is rapidly converted in the body to oxalic acid, which can cause irreversible kidney damage. However, the active site of the enzyme which converts ethylene glycol to oxalic acid will also accept ethanol. If the poisoned person is given a large dose of ethanol, the ethanol acts as a competitive inhibitor, slowing down the action of the enzyme on ethylene glycol for long enough to allow the ethylene glycol to be excreted.
Inhibition of enzyme function can be lethal, but in many situations inhibition is essential. For example, metabolic reactions must be very finely controlled and balanced, so no single enzyme can be allowed to 'run wild', constantly churning out more and more products.
One way of controlling metabolic reactions is to use the end-product of a chain of reactions as a non-competitive, reversible inhibitor. As the enzyme converts substrate to product, it is slowed down because the end product binds to another part of the enzyme and prevents more substrate binding. However, the end-product can lose its attachment to the enzyme and go on to be used elsewhere, allowing the enzyme to reform into its active state. As product levels fall, the enzyme is able to top them up again. This is termed end-product inhibition.
Q.1. What are enzymes?
Ans: Enzymes are biological catalysts mainly made of proteins and can alter the rates of chemical reactions without themselves being chemically changed at the end of the reaction.
Q. 2. What are the functions of enzymes?Ans: Characteristics/functions of enzymes-a) speed up chemical reactionsb) highly specificc) catalyse reactions under relatively mild conditionsd) may need coenzymes for activitye) digest food substancesf) build-up or synthesize complex substances e.g. proteins from amino acids in cellsg) break down food substances in cells to release energy (cellular respiration)h) control the numerous reactions in an organism
Q.3. What are the advantage of industrial applications of enzymes?
Ans: Enzymes are very useful substances which industrial chemists would also like to use since they are able to bring about chemical changes at low temperatures. Industrial enzymes are enzymes that are commercially used in a variety of industries such as pharmaceuticals, chemical production, food, beverage and consumer products.
The advantages of industrial applications of enzymes are -a) Way of using enzymes is to use whole organisms in industrial processes. For example, the use of micro-organisms to make cheese, yogurt and beer.b) Diabetics can test urine for the presence of glucose using a clinistix. It contains two enzymes: glucose oxidase and peroxidase. Glucose oxidase breaks down glucose to produce to hydrogen peroxide. This hydrogen peroxide is then combined with a dye in the stick by the second enzyme, peroxidase.c) They are specific so they only catalyse the reaction.d) Using lower temperatures and pressures means a lower cost as it saves energy.e) They are biodegradable and therefore cause less environmental pollution.
Digestion in the small intestine:
When the chyme enters the duodenum, two secretions, pancreatic juice from the pancreas and bile from the liver, are released through the bile from the pancreas, and bile from the liver, is released through the bile duct. Both of these secretions are alkaline in nature. Pancreatic juice neutralizes the acidity of the chyme. The enzymes of the pancreatic juice continue the digestion process of proteins and carbohydrates and start fat digestion. Bile neutralizes the acidity of the food and creates an alkaline medium. Bile salt, one of the constituents of bile, emulsifies fats, meaning it helps fat droplets to mix with water. It then becomes easier for the enzyme lipase to digest fats. The Lipase converts the fat droplets into fatty acids and glycerol.
Fats ----------------------Lipase-------- Fatty acid + glycerolPancreatic juice contains trypsin, lipase and amylase. On the other hand, intestinal juice contains the enzymes, maltase, lactose, and sucrose. Trypsin, converts partly digested protein into amino acid and simple peptide.Polypeptide ------------------Trypsin -------------- Amino acid + simple peptideAmylase converts starch into simple glucose.Starch --------------amylase------ glucose
Q.3. What are immobilised enzymes?
Ans: Enzymes that have been fixed to a surface or within a bead of agar gel.
Q.4. Why enzymes are important?
Ans: Enzymes have an enormous range of commercial applications - for example, in medicine, food technology and industrial processing. Enzymes are expensive. No company wants to have to keep buying them over and over again if it can recycle them in some way. One of the best ways of keeping costs down is to use immobilised enzymes.
Q.5. How we can immobilised enzymes?
Ans: The enzyme lactose can be immobilised using alginate beads. Milk is then allowed to run through the column of lactose-containing beads. The lactose hydrolysis the lactose in the milk to glucose and galactose. The milk is therefore lactose-free and can be used to make lactose free dairy products for people who can not digest lactose.
Process:
The enzyme is mixed with a solution of sodium alginate. Little droplets of this mixture are then added to a solution of calcium chloride. The sodium alginate and calcium chloride instantly react to form jelly, which turns each droplet into a little bead. The jelly bead contains the enzyme. The enzyme is held in the bead, or immobilised.
These beads can be packed gently into a column. A liquid containing the enzyme's substrate can be allowed to trickle steadily over them.
As the substrate runs over the surface of the beads, the enzymes in the beads catalyse a reaction that converts the substrate into product. The product continues to trickle down the column, emerging from the bottom, where it can be collected and purified.
Factors that affect enzyme action:
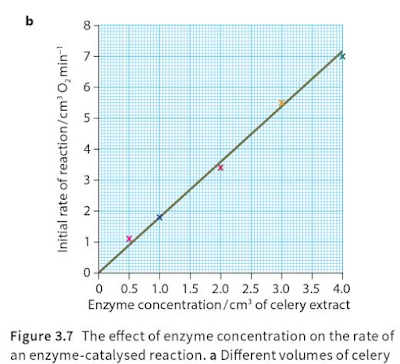
The effect of enzyme concentration:
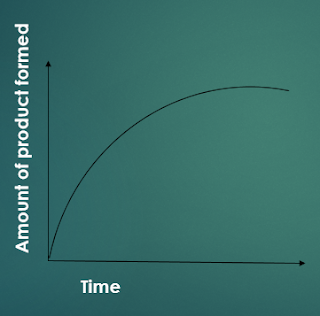
This is a diagram shows the results of an investigation in which different concentrations of catalase solution were added to the same volumes of hydrogen peroxide solution. Concentration was varied by varying the initial volume of extract and then making up to a standard volume. The shape of all five curves is similar. In each case, the reaction begins very quickly and then gradually slows down. Because the quantity of hydrogen peroxide is the same in all five reactions, the total amount of oxygen eventually produced will be the same; so, if the investigation goes on long enough, all the curves will meet.
To compare the rates of these five reactions, in order to look at the effect of enzyme concentration on reaction rate, it is fairest to look at the rate right at the beginning of the reaction. This is because, once the reaction is underway, the amount of substrate in each reaction begins to vary, because substrate is converted to product at different rates in each of the five reactions. It is only at the very beginning of the reaction that we can be sure that differences in reaction rate are caused only by differences in enzyme concentration.
To work out the initial rate for each enzyme concentration, we can calculate the slope of the curve 30 seconds after the beginning of the reaction, as explained earlier. Ideally, we should do this for an even earlier stage of the reaction, but in practice this is impossible. We can then plot a second graph, Figure showing the initial rate of reaction against enzyme concentration.
This graph shows that the initial rate of reaction increases linearly. In these conditions, the reaction rate is directly proportional to the enzyme concentration. This is just what common sense says should happen. The more enzymes present, the more active sites will be available for the substrate to slot into. As long as there is plenty of substrates available, the initial rate of a reaction increases linearly with enzyme concentration.
Q.3.1 a. In the breakdown of starch by amylase, if you were to plot the amount of starch remaining against time, sketch the curve you would expect to obtain.b. How could you use this curve to calculate the initial reaction rate?
3.2. Why is it better to calculate the initial rate of reaction from a curve such as the one in figure 3.6, rather than simply measuring how much oxygen is given off in 30 seconds?
The significance of Vmax and Km values:1. It enables scientists to make computerised models of biochemical pathways or even the behaviour of whole cells because it helps to predict how each reaction in a proposed pathway will proceed and therefore how the enzymes will interact. the consequences of changing conditions such as temperature, pH or the presence of inhibitors can be built into the models.
2. An enzyme's preference for different substrates can be compared quantitatively.3. By understanding what affects enzyme efficiency, scientists may in future be able to design better catalysts, linking this to genetic engineering.
4. For a commercially important enzyme, the performance of the same enzyme from different organisms can be compared.
5. The calculations involved can be applied to other fields of biochemistry, such as antibody - antigen binding.
6. Knowing Km means the proportion of active sites occupied by substrate molecules can be calculated for any substrate concentration.
Q.3.9. Which of the four enzymes in table 3.2 has the highest affinity for its substrate? Briefly explain your answer.
Q.5. What are the advantages of immobilised enzymes?
Ans: Enzyme immobilization has several obvious advantages compared with just mixing up the enzyme with its substrate. If we just mixed lactose with milk, you would have a very difficult task to get the lactose back again. Not only would you lose the lactose, but also you would have milk contaminated with the enzyme. Using immobilised enzymes means that you can keep and re-use the enzymes, and that the product is enzyme -free.
Another advantage of this process is that the immobilised enzymes are more tolerant of temperature changes and pH changes than enzymes in solution. This may be partly because their molecules are held firmly in shape by the alginate in which they are embedded, and so do not denature as easily. It may also be because the parts of the molecules that are embedded in the beads are not fully exposed to the temperature or pH changes.
Lock and Key and induced fit hypotheses:
Ans: Enzymes are globular proteins. Enzyme molecules have a special feature in that they possess an active site. The shape of the active site allows the substrate to fit perfectly. The idea that the enzyme has a particular shape into which the substrate fits exactly is known as the lock and key hypothesis.
The substrate is the key whose shape fits the lock of the enzyme. The substrate is held in place by temporary bonds which form between the substrate and some of the R groups of the enzyme's amino acids. This combined structure is termed the enzyme- substrate complex.
Each type of enzyme will usually act on only one type of substrate molecule. This is because the shape of the active site will only allow one shape of molecule to fit. The enzyme is said to be specific for this substrate.
An enzyme may catalyse a reaction in which the substrate molecule is split into two or more molecules. Alternatively, it may making a dipeptide. Interaction between the R groups of the enzyme and the atoms of the substrate can break, or encourage formation of, bonds in the substrate molecule, forming one, two or more products.
When the reaction is complete, the product or products leave the active site. The enzyme is unchanged by this process, so it is now available to receive another substrate molecule. The rate at which substrate molecules can bind to the enzyme's active site, be formed into products and leave can be very rapid. The enzyme catalase, for example, can bind with hydrogen peroxide molecules, split them into water and oxygen, and release these products at a rate of 10 million molecules per second.
The interaction between the substrate and the active site, including the slight change in the shape of the enzyme which results from the binding of the substrate, is clearly shown by the enzyme lysozyme. Lysozyme is a natural defense against bacteria that is found in tears, saliva and other secretions. It breaks the polysaccharide chains that form the cell walls of bacteria.


















Comments