Biological Molecules
- In biology, certain molecules are essential for life.
- These molecules—carbohydrates, proteins, lipids and nucleic acids —are the building blocks of cells and are involved in nearly every function that supports life.
- Simplified versions are shown opposite.
Learning Objects:
* How large biological molecules are made from smaller molecules
* Describe the structure & function of carbohydrates, lipids and proteins
* Carry out biochemical tests to identify carbohydrates, lipids & proteins
* Explain some key properties of water that make life possible.
Q.1. What is macromolecule?
Ans: A macromolecule is a large biological molecule such as a protein, polysaccharide or nucleic acid. There are 3 types of macromolecule in living organisms-
1) polysaccharides
2) proteins
3) nucleic acids
Q.2. What is monomer?
Ans: A monomer is a relatively simple molecule which is used as a basic building block for the synthesis of a polymer; many monomers are joined together to make the polymer, usually by condensation reactions; common examples of molecules used as monomers are monosaccharides, amino acids and nucleotides.
Q.3. What is polymer?
Ans: A polymer is a giant molecule made from many similar repeating subunits joined together in a chain; the subunits are much smaller and simpler molecules known as monomers; examples of biological polymers are polysaccharides, proteins and nucleic acids.
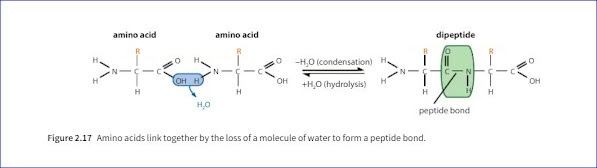
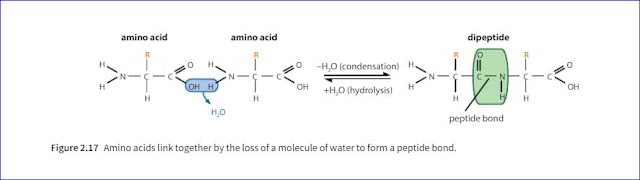
4. What is peptide bond?
Ans: One loses a hydroxyl (-OH) group from its carboxylic acid group, while the other loses a hydrogen atom from its amine group. This leaves a carbon atom of the first amino acid-free to bond with the nitrogen atom of the second. The link is called a peptide bond.
Q.5. What are carbohydrates?
Ans: Carbohydrates are organic compounds made up of the elements carbon, hydrogen and oxygen. Carbohydrates are divided into 3 main groups, namely.
a) monosaccharides,
b) disaccharides and
c) polysaccharides.
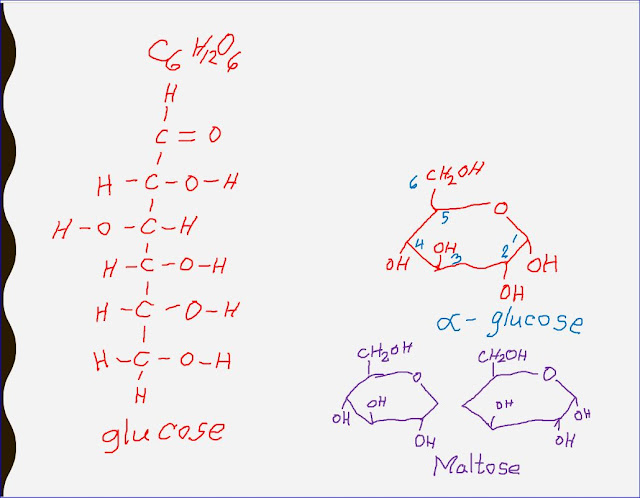
Q.2.1. The formula for a hexose is C6H12O6 or (CH2O)6.
What would be the formula of:
a. a triose (3C)
b. a pentose (5C)?
Q.6. What is the difference between monosaccharides, disaccharides and polysaccharides?
Glycosidic bond:
For each condensation reaction, two hydroxyl (-OH) groups line up alongside each other. One combines with a hydrogen atom from the other to form a water molecule. This allows an oxygen 'bridge' to form between the two molecules, holding them together and forming a disaccharide. The bridge is called a glycosidic bond.
In theory any two -OH groups can line up and, since monosaccharides have many -OH groups, there are a large number of possible disaccharides. The shape of the enzyme controlling the reaction determines which -OH groups come alongside each other. Only a few of the possible disaccharides are common in nature.
The reverse of condensation is the addition of water, which is known as hydrolysis. This takes place during the digestion of disaccharides and polysaccharides when they are broken down to monosaccharides.
Simple Sugar or Monosaccharides:
There are numerous simple sugars. The most common ones are the sugars with six carbon atoms, with the general formula C6H12O6 These sugars- glucose, fructose and galactose-differ only in details of the arrangement of the various atoms within the molecule which give them different chemical and biological properties.
Glucose (grape sugar) is found, at least in small quantities, in all animals. Fructose is widespread in plants but uncommon in animals. Galactose is a component of milk sugar or lactose is rather rare in organisms other than mammals.
Alcohols are a series of organic molecules which contain a hydroxyl group, -OH, attached to a carbon atom. Glycerol is an alcohol with three hydroxyl groups.The reaction between an acid and an alcohol produces a chemical known as an ester. The chemical link between the acid and the alcohol is known as an ester bond or an ester linkage.The -COOH group on the acid reacts with the -OH group on the alcohol to form the ester bond, -COO-. This is a condensation reaction because water is formed as a product. The resulting ester can be converted back to acid and alcohol by the reverse reaction of adding water, a reaction known as hydrolysis.
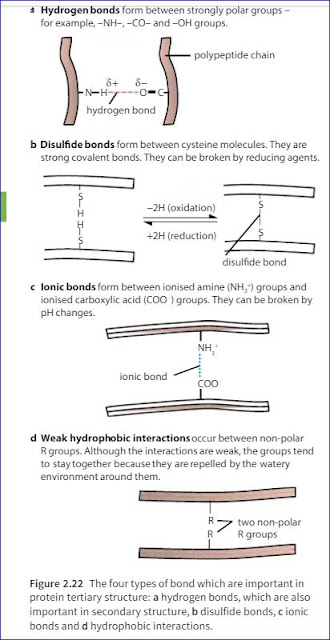
Amino acids have a central carbon atom which is bonded to an amine group, -NH2, and a carboxylic acid group, -COOH. It is these two groups which give amino acids their name. The third component that is always bonded to the carbon atom is a hydrogen atom.The only way in which amino acids differ from each other is in the remaining, fourth, group of atoms bonded to the central carbon. This is called the R group. There are 20 different amino acids which occur in the proteins of living organisms, all with a different R group.
Functions of Carbohydrates:
1. As a source of energy.
2. To form supporting structures e.g. cellulose cell walls in plants.
3. To be converted to other organic compounds such as amino acids and fats.
4. For the function of nucleic acids (e.g. DNA).
5. To synthesize lubricants, e g. mucus which consists of a carbohydrate and a protein. The mucus lining the respiratory system in Man traps dust particles.
6. To produce the nectar in some flowers. The sugary nectar attracts feeding insects and enables cross-pollination to occur.
Condensation Reaction:
The smaller units are joined together by condensation reactions. Condensation involves the removal of water. The reverse process, adding water, is called hydrolysis and is used to break the large molecules break down into smaller molecules.
Condensation is a chemical reaction whereby two simple molecules are joined together to form a larger molecule with the removal of one molecule of water.
Condensation reactions are very important in the formation of large polymers such as starch and proteins. Condensation is the reverse of hydrolysis.
Q.1. What are fats?
Ans: Fats are organic compounds made up of the elements carbon, hydrogen and oxygen but, unlike carbohydrates, they contain much less oxygen in proportion to hydrogen.
Q.2. What are the functions of fats?
Ans: The functions of fats are-
a) As an efficient source and storage of energy.
b) As an insulating material. especially beneath the skin, to prevent excessive heat loss. In mammals that live in the water, there is a greater tendency to lose heat since the hair cover is no longer an efficient insulator in water. Thus, animals such as whales have a thick layer of blubber beneath the skin which helps to retain body heat.
c) As a solvent for fat-soluble vitamins and many other vital substances, including sex hormones and related hormones.
d) As a constituent of protoplasm, especially in the protoplasmic membranes.
e) As a means to restrict water loss from the surface of the skin. The oily secretion produced by sebaceous glands in the skin forms a thin layer over the surface, thus reducing the rate of evaporation of water. This also reduces the rate of heat loss from the skin.
Q.3. Is there any difference between fats and oils?
Q.4. Who should be avoided fats in the diet?
Q.5. What is the difference between saturated and unsaturated fats?
Hydrolysis Reaction:
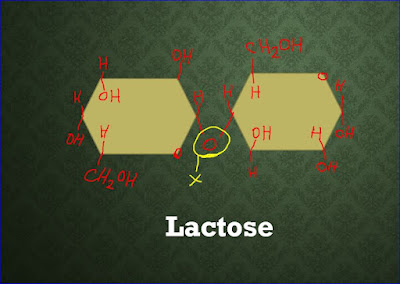
Ans: Lactose is the sugar found in milk and is therefore an important constituent of the diet of young mammals.
Ans: The joining of two monosaccharides takes place by a process known as condensation. Two molecules of glucose and galactose combine to make the disaccharide lactose. Lactose consisting of two monosaccharides joined together by a glycosidic bond.
8. a. The diagram below shows the structures of three amino acids.
alpha-globin
beta-globin
What is Globular protein?
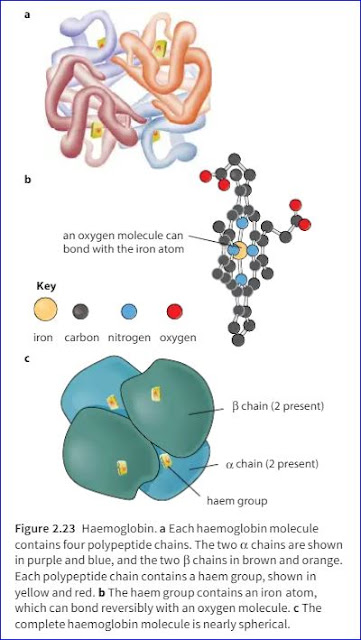
Ans: A protein whose molecules curl up into a ‘ball’ shape, such as myoglobin or haemoglobin, is known as globular protein. Haemoglobin is the oxygen-carrying pigment found in red blood cells and is a globular protein.
Water:
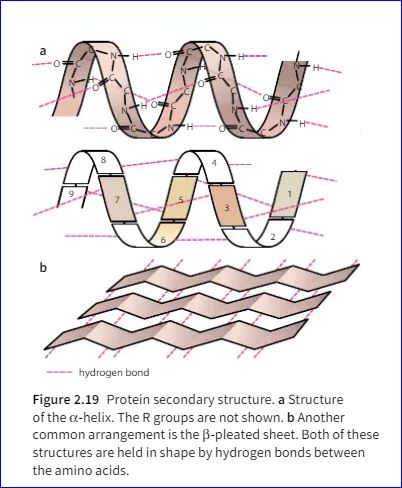
The hydrogen bonding of water molecules makes the molecules more difficult to separate and affects the physical properties of water. For example, the energy needed to break the hydrogen bonds makes it more difficult to convert water from a liquid to gas than to convert similar compounds which lack hydrogen bonds, such as hydrogen sulfide (H2S), which is a gas at normal air temperatures.
Water is an excellent solvent for ions and polar molecules because the water molecules are attracted to the ions and polar molecules and therefore collect around and separate them. This is what happens when a chemical dissolves in water. Once a chemical is in solution, it is free to move about and react with other chemicals. Most processes in living organisms take place in solution in this way.

























Comments